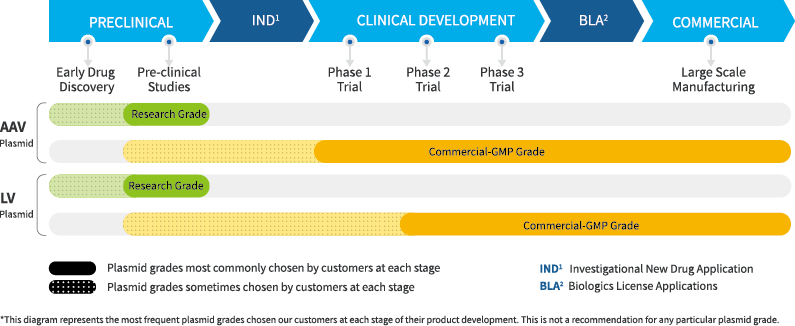
Our patented plasmids achieve high-titre, high quality viral vector production. XLenti™ plasmids can improve titers 10-fold compared to other commercially available plasmids, and our reconfigured XAAV™ plasmids improve titers up to 8 fold (depending on serotype) compared to standard RepCap plasmids.
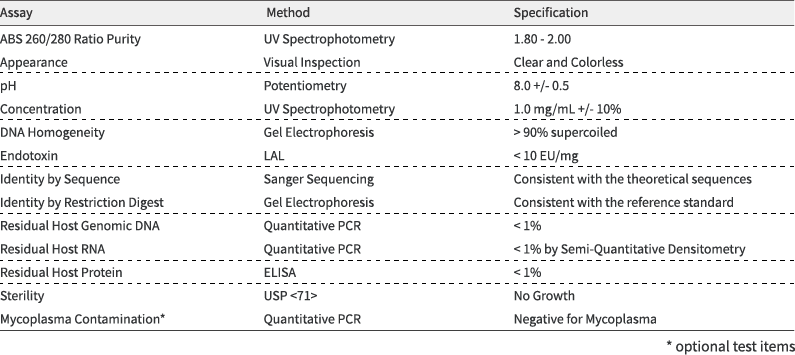
We provide Letter of Authorization to use our Drug Master File to accelerate your US FDA Investigational New Drug (IND) & Biologics License Application (BLA) processes. Also, CTD Module 3 can be developed to support your China NMPA’s CDE IND & BLA processes. Our test methods are fully validated per ICH and other regulatory guidelines.
Our antibiotics free manufacturing process is highly preferred by regulatory agencies. It means that further testing to measure and control for residual antibiotics is not required. Our bioreactors and consumables are single use and animal component free to eliminate microbial contamination.
We have filed over 25 patent applications for our plasmid technology globally. Both off-the-shelf and custom plasmids are available royalty free from research to commercial use.

| Plasmid Type | Product Code | Description | Pack Size |
| AAV Plasmid | AQ6972, AQ6973, AQ6975 | Rev、VSVG、GagPol | 1mg, 10mg |
| AAV Plasmid | AQ1364 | AAV Helper | |
| AQ6587 | AAV1 Rep-Cap | ||
| AQ6801 | AAV2 Rep-Cap | ||
| AQ6589 | AAV3 Rep-Cap | ||
| AQ6802 | AAV4 Rep-Cap | ||
| AQ5220 | AAV5 Rep-Cap | ||
| AQ6961 | AAV6 Rep-Cap |
 Contact us for product inquiries
Contact us for product inquiries
Download Zone
