The quality management system of WuXi ATU is established based regulations from major countries, including:

Method development and validation, complying with ChP, USP, PhEur and relevant guidelines
Release testing of in-process control samples and final products
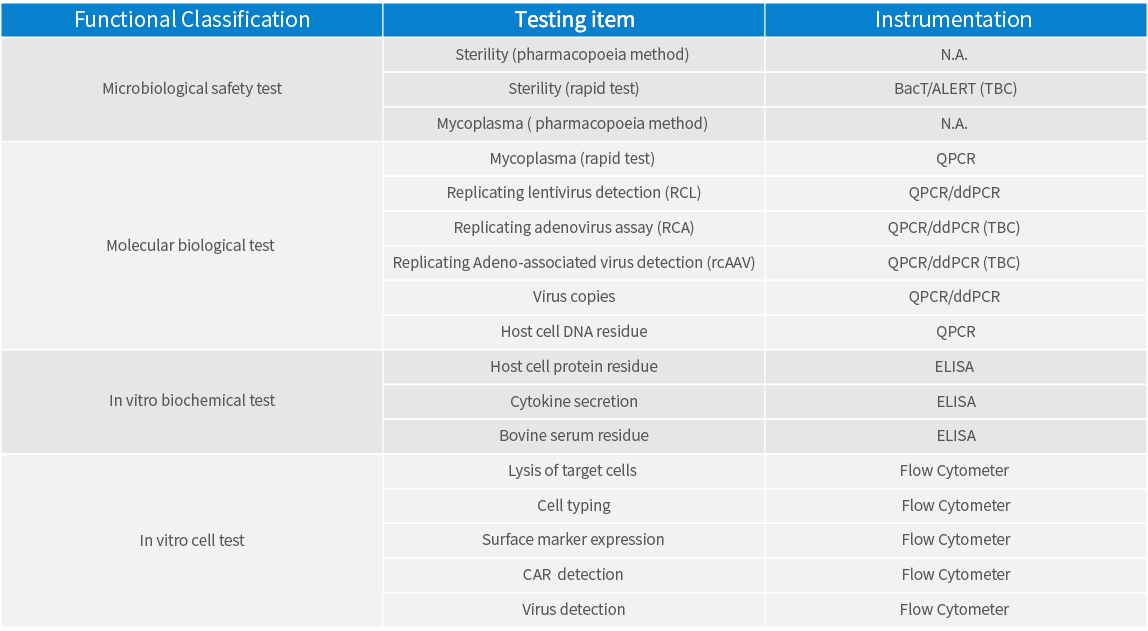
Sound quality standards, covering bacterial banks, cell banks, plasmid vectors, viral vectors, CAR-T products, etc.
One-stop service for stability study protocol, execution and report, meeting the requirements of customer application and also complying with ICH Q1A/B
Individual contract testing services, such as sterility testing, and mycoplasma testing, etc.

Analytical method development for special testing
Comprehensive and sufficient analytical method development
Comprehensive analytical method validation system
Precise and efficient test services
Comprehensive testing capability