The CTDMO platform for cell therapy of WuXi ATU has a human genetic material management system, in meeting regulatory requirements, providing immune cell therapy products, meeting cGMP production requirements for customers worldwide, accelerating the review for approval, and marketing of cell therapy products.

CAR-T cells

TCR-T cells

CAR-NK cells

DC immune cells

Planning and design of immune cell product development route based on QbD concept
Industrialization-oriented immune cell process development and scale-up
Immune cell product quality control and strategy consultation
Analytical method development and quality study meeting the requirements for external application
Preparation of pharmaceutical study and IND application dossier of immune cell products
Production of clinical trial samples and large-scale commercial production

The CTDMO platform of WuXi ATU cell therapy products is planned and designed based on the QbD concept. With BSL-2 level biosafety, independent air-conditioning full exhaust system and disposable and fully enclosed flexible production process, it effectively prevents contamination and cross contamination while reducing the cost of large-scale production.
Static culture, stirring culture and swing culture and other flexible cell amplification methods meet the needs of customers at different scales
A complete quality management system with complete production traceability and compliance provides customers with more rapid, convenient, safe and effective immune cell therapy products
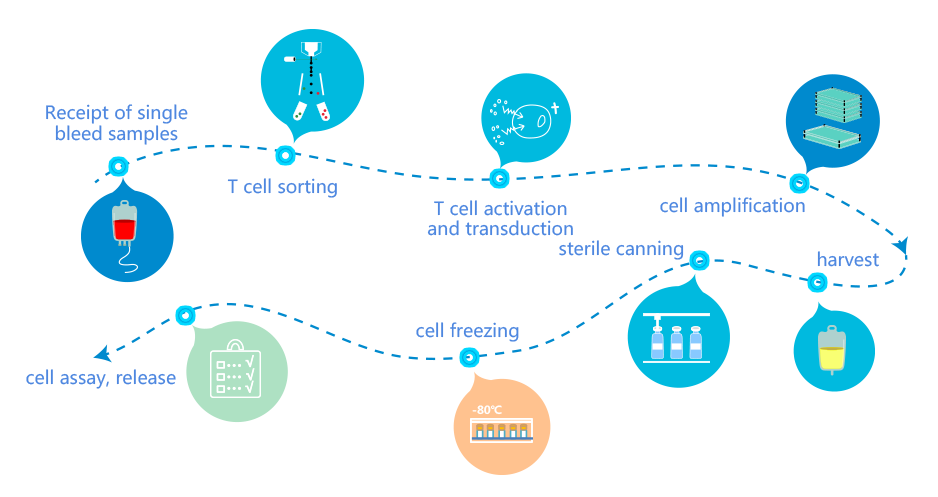
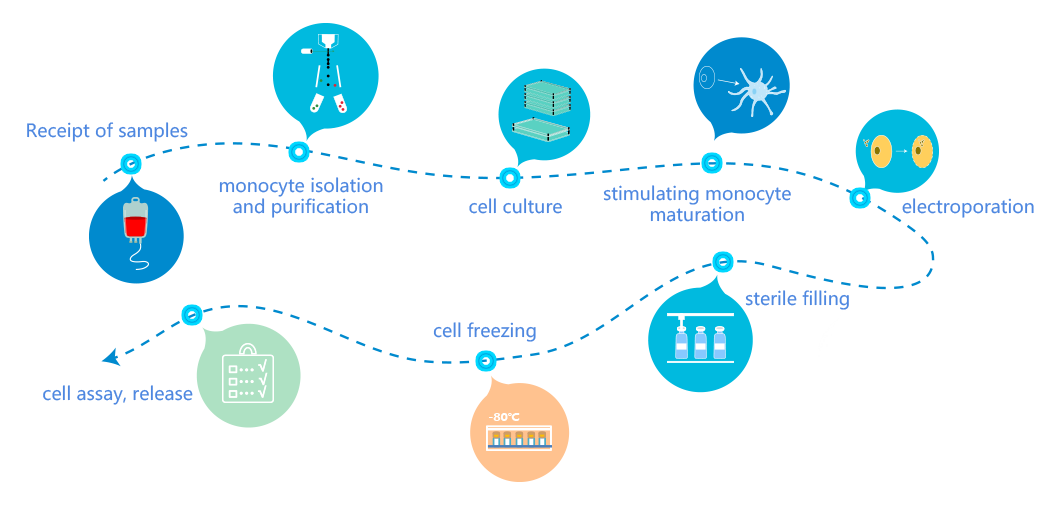
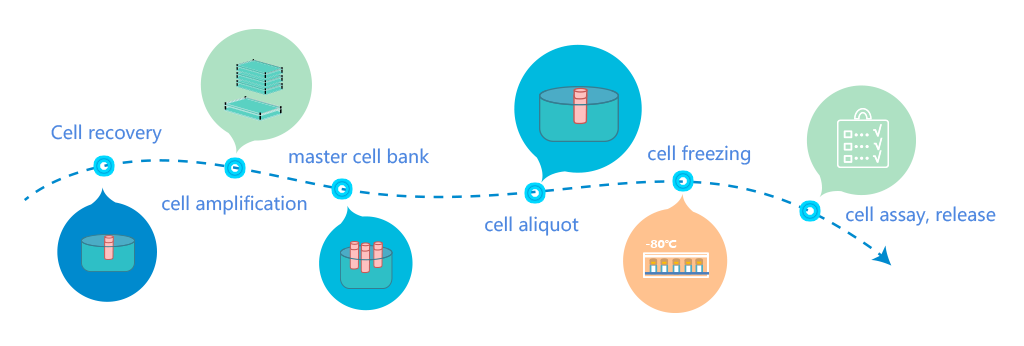

BSL-2 level biosafety, independent air conditioning system, full exhaust clean background environment
Fully enclosed disposable sterile production process, separate personnel flow, sample flow and material flow, one-way flow design, to minimize contamination and cross contamination
Modular design, flexible arrangement of production, to meet the needs of different scales of production full process traceability, safe and reliable
Human genetic material management system meeting the regulatory requirements
