



The design based on the QbD concept and a fully enclosed system, separation of human flow and logistics, disposable equipment and processes help effectively reduce contamination and cross-contamination
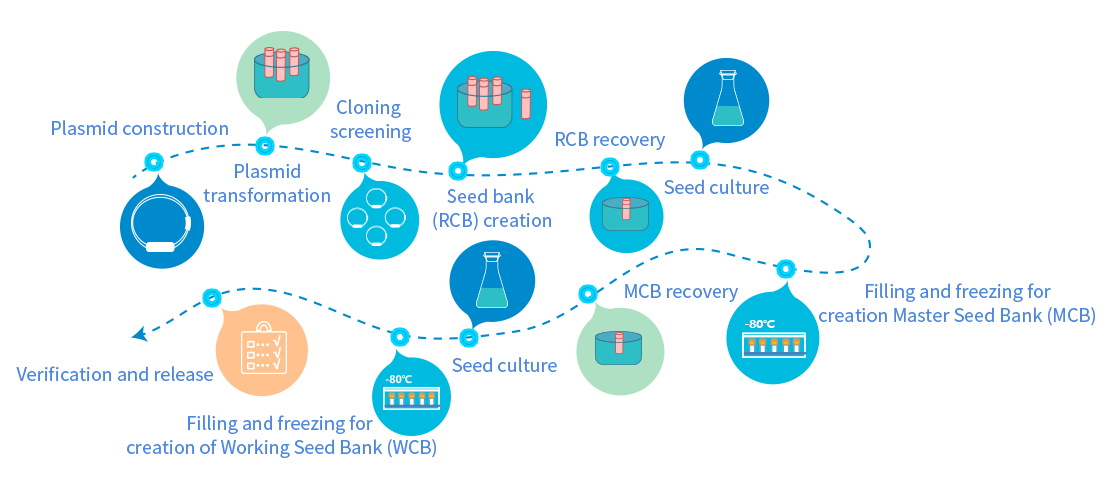
An independent plasmid development laboratory, a flexible production scale, and culture with a shaker flask or fermentor meet different customer needs
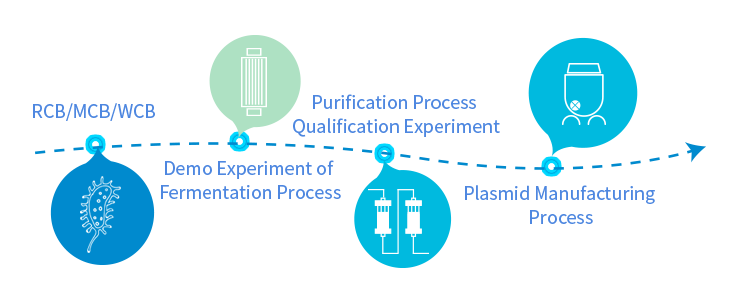
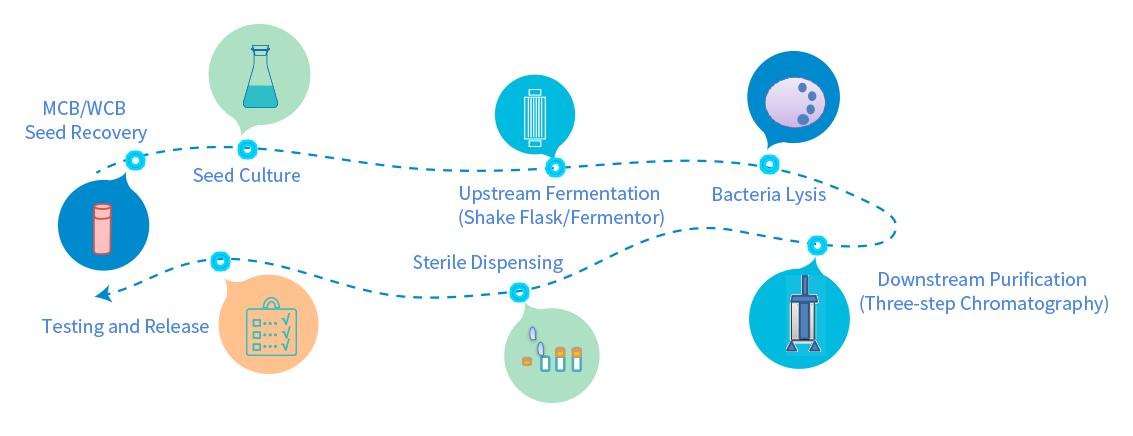
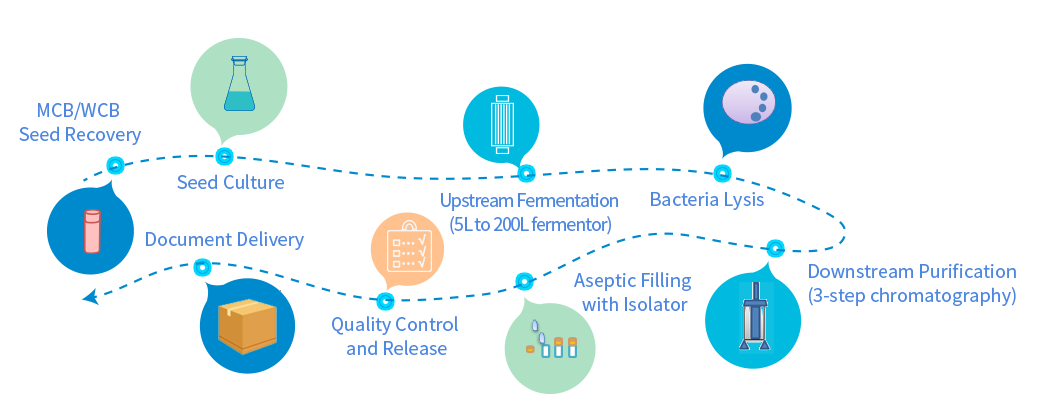
Independent GMP manufacturing workshop, high-density fermentation process, and control of the whole production process meet the GMP manufacturing requirements of China, the United States and the European Union
On-time delivery contributes to a significant price competitive advantage
A professional, efficient and experienced team enables full process service
The well-developed quality management system helps to successfully achieve the audit and cooperation of many pharmaceutical partners worldwide
