The CTDMO platform for lentiviruses and adeno-associated virus vectors of WuXi ATU consists of five open production technology platforms that can efficiently provide high-quality services with price advantage to customers worldwide. Based on the QbD concept and cGMP requirements of the United States, European Union and China, WuXi ATU has designed and built four independent viral vector GMP manufacturing lines (2,100 m2), which have passed the audit by a number of pharmaceutical partners with achievement of successful cooperation.

293T/293 cells and insect cells (suspended and adherent cell types) with clear sources and clear authorization are used on the platform, which are stable and uniform. A three-level GMP cell bank system has been established for all cell lines, and they are tested and released according to the requirements of pharmacopoeias.
It can provide customers with high-quality cell bank construction services in line with the GMP requirements, such as three-level cell bank construction, cell passage stability study, etc., and provide complete relevant experimental records.
Provide customers with high-quality cell products for viral vector production to help customers accelerate their product development.

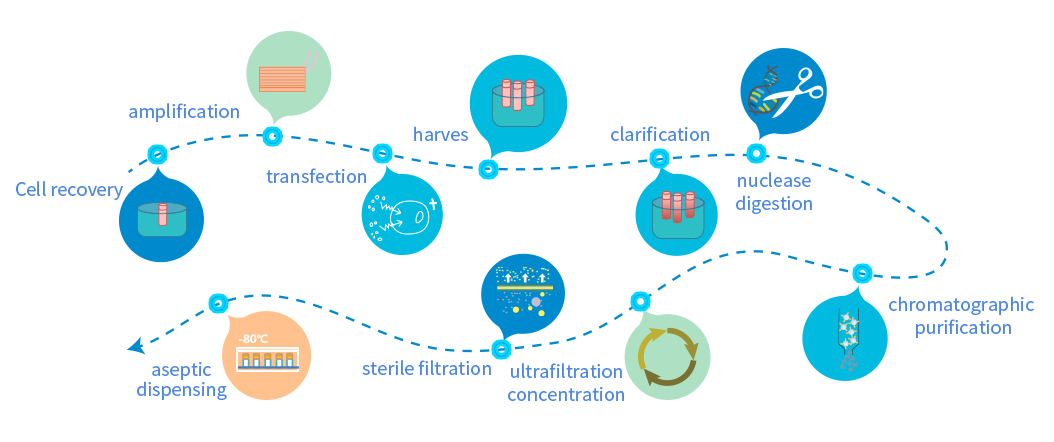

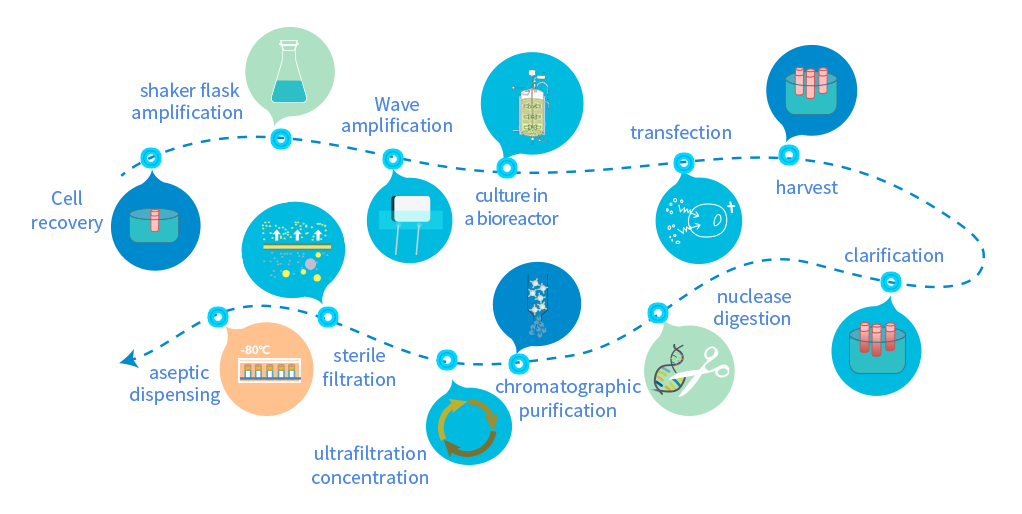

Equipped with a BSL-2 Grade GMP Viral Vector Production Workshop, and a Grade A Sterile Isolator Filling System
Advanced fully enclosed disposable aseptic manufacturing process with separate personnel flow and one-way flow design to minimize contamination and cross-contamination
8 independent production operation units of modular design ensure flexible production scheduling with a production capacity up to 120 batches/year
A complete quality control system enables strict monitoring and release of the production process, with a complete and traceable CMC original record
A professional technical production and project management team with rich experience in large-scale production, to achieve full-process tracking management by special personnel
Meet the requirements of cGMP production management system in China, the United States and Europe, and have provided CTDMO services to customers in many countries around the world
