LentiVV Stable | New Generation LVV Production Technology2023年8月23日
In recent years there has been an emergence of safety and efficacy data for cell and gene therapy (CGT), providing new possibilities for the treatment of various diseases. Lentivirus is one of the widely used viral vectors in cell therapy field, and the mainstream production system is the third-generation lentiviral vector production system – the four-plasmid transient transfection system, which requires GOI, Rev, GagPol and VSV-G co-transfection into HEK293 cell.
In the four-plasmid transient transfection system, the vector manufacturing process is facing the challenge of high costs, with plasmid DNA accounting for the highest proportion of cell therapy drug development. GMP-compliant plasmid DNA can cost tens of thousands of dollars per gram, accounting for 18%-46% of production costs in different processes at a commercial production scale[1]. According to the comparative analysis of the material costs of five upstream processes for producing lentiviral vectors, in the case of a production yield of 1000 doses per year, the cost of plasmid DNA accounts for the highest proportion among all the material costs, ranging from 36%-46%, in the large-scale processes suitable for commercial stage manufacture, such as fixed-bed (FB333), microcarrier (RMmc600), and stirred-tank (SUB500) bioreactors.
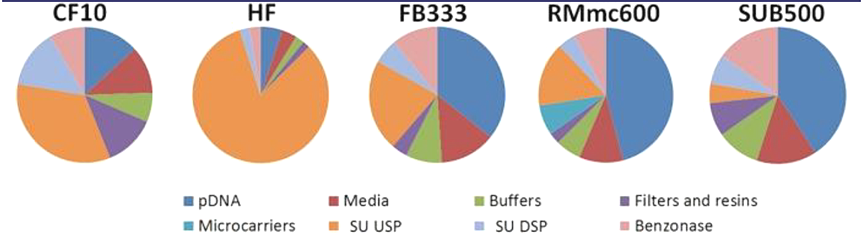 Data source: Biochemical Engineering Journal
Therefore, there is an urgent need for a disruptive production solution for lentiviral vectors to reduce the cost per dose and enable more products to enter clinical and commercial stages, ultimately becoming accessible to more patients worldwide. The stable lentiviral packaging and producer cell line technology is becoming an important direction of the cell therapy industry due to its scalability and lower cost.
As a global innovative CTDMO (Contract Testing, Development and Manufacturing Organization), WuXi Advanced Therapies (WuXi ATU) has developed the stable LentiVV Packaging Cell Line and the LentiVV Producer Cell Line starting from a GMP banked highly productive HEK293 suspension cell line, which was originally selected through high-throughput screening from thousands of single cell clones.
The LentiVV Packaging Cell Line integrates the components for lentivirus packaging, such as Rev, GagPol, and VSV-G, into the genome of suspension HEK 293 cells. The Envelope (VSV-G) and GagPol elements are controlled by a Tetracycline-Regulated promoter; the system requires induction by addition of inducing agent (doxycycline) to produce LVV. Compared to the traditional four-plasmid transient transfection system, the LentiVV Packaging Cell Line requires transient transfection of only one plasmid, significantly reducing plasmid production costs and process development complexity.
The LentiVV Producer Cell Line, developed based on the Packaging Cell Line, integrates the GOI transfer plasmid into the genome of the Packaging Cell Line, providing a plasmid-free method for lentiviral vector production. This approach saves on plasmid and transfection reagent costs, increases scalability to large bioreactor scale (500-1000L) due to absence of transient transfection step, and significantly improves process robustness.
Both the LentiVV Packaging Cell Line and Producer Cell Line can achieve titers comparable to or even higher than the four-plasmid transient transfection. (Table 1, Figure 2).
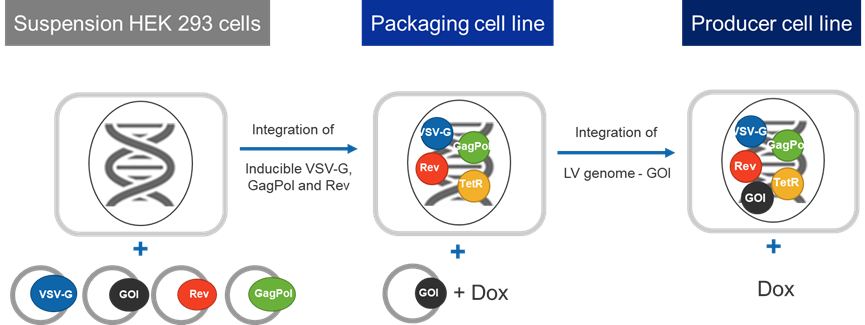
Fig 1. Technical Principle of LentiVV Packaging Cell Line & LentiVV Producer Cell Line
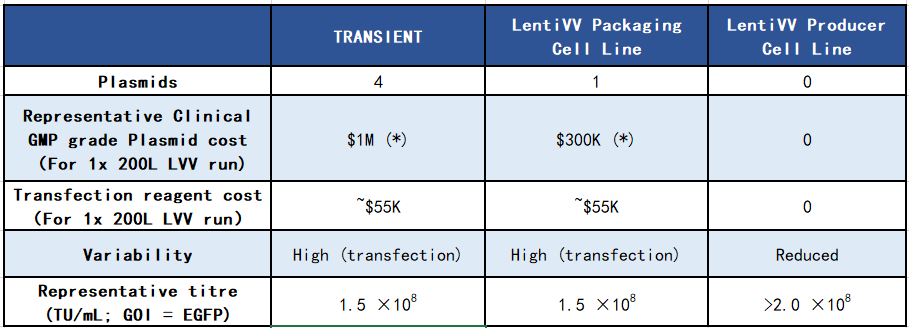 Tab 1. Comparison between four-plasmid transient transfection system and LentiVV Packaging Cell Line, LentiVV Producer Cell Line 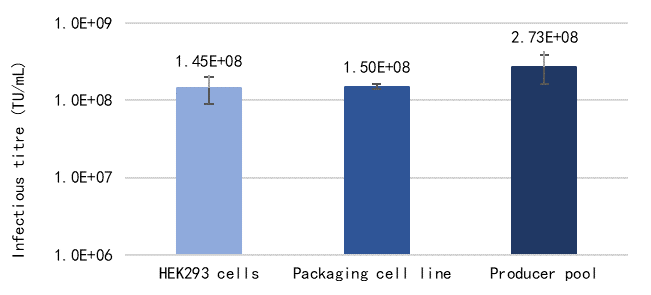 Fig 2. Lentiviral vector production capability comparison between LentiVV Packaging Cell Line, LentiVV Producer Cell Line and four-plasmid transient transfection system(GOI=GFP,E125 flask scale
LentiVV Packaging Cell Line
Only One Plasmid Needed,
Suitable for Early Stage Gene Screening and Vector Development
WuXi Advanced Therapies LentiVV Packaging Cell Line (VSV-G pseudotype) was selected among hundreds of clonal cell lines based on criteria such as growth rate, efficiency of transfection and LVV productivity. The platform retains stability over 27 passages (~ 88 doublings), based on growth rate, integrated gene copy number and LV productivity. (Figure 3)
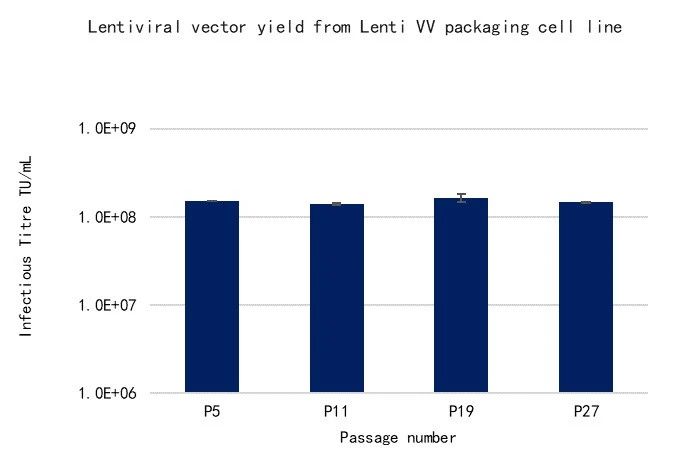 Figure 3: Stability testing of the packaging cell line, showing consistent yields over 27 passages (P). Consistent yields across multiple passages are used as a proxy measure to confirm genome stability.
Comparative studies between the Packaging Cell Line and the four-plasmid transient transfection system shows that for different GOIs, the titer of lentivirus produced by the Packaging Cell Line are equivalent to or higher than four-plasmid transient transfection system (Figure 4).
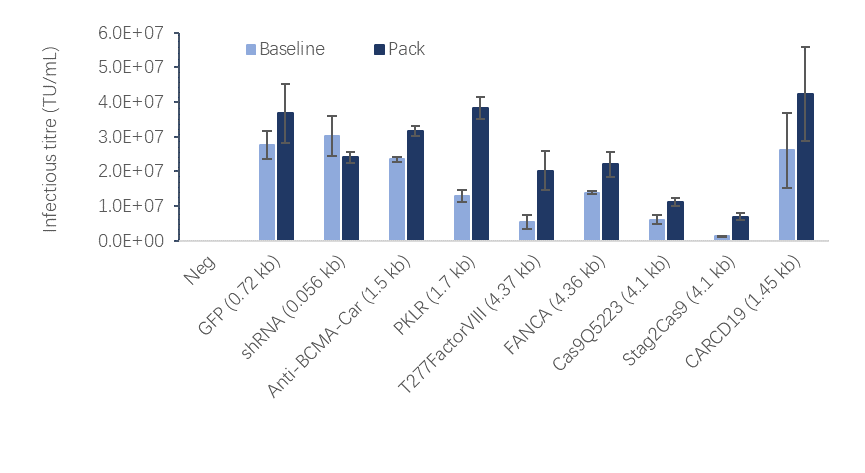
Fig 4. Lentiviral vector production capability comparison between LentiVV Packaging (Pack) Cell Line and four-plasmid transient transfection system
LentiVV Producer Cell Line
No Plasmid Transfection Required, Suitable for Large-Scale Production, Significantly Reducing Costs
By integrating the GOI transfer plasmid into the LentiVV Packaging Cell Line using transposons, WuXi Advanced Therapies has developed the LentiVV Producer Cell Line that eliminates transient transfection and is more suitable for large-scale production. We demonstrated feasibility of LentiVV Producer Cell Line Development approach by successful integration of 8 therapeutically relevant GOIs, which differ in size and functionality, and LVV production at the stable pool stage (heterogeneous population). The use of LentiVV Producer Cell Line to produce lentiviral vectors for different target genes has achieved high titer of >3×107 TU/mL (Fig 5). We also submitted GFP and Anti-CAR-CD19 stable LentiVV Producer Pools for clonal cell line isolation and, through multiple rounds of screening, the best performing clonal cell lines were identified to enter Process Development studies.
In terms of its production capability, scale-up studies have shown that the titers achieved in bioreactors are comparable to those in E125 flasks, demonstrating the excellent scalability (Fig 6).
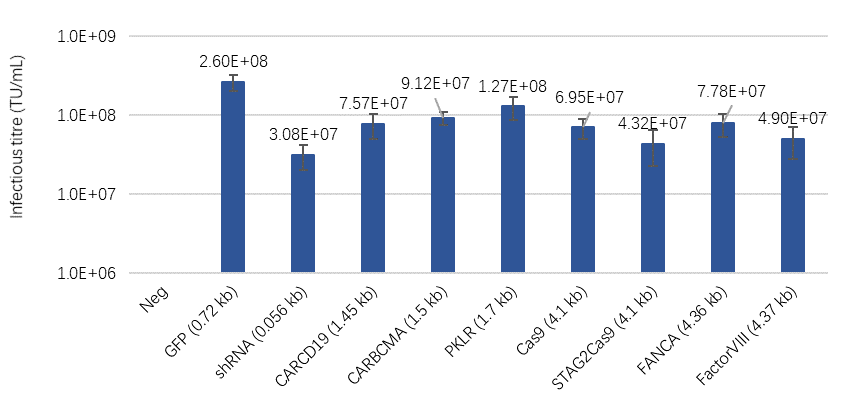
Fig 5. Titer of lentiviral vector for different GOIs produced from LentiVV Producer Pools(24 deep-well plate scale)
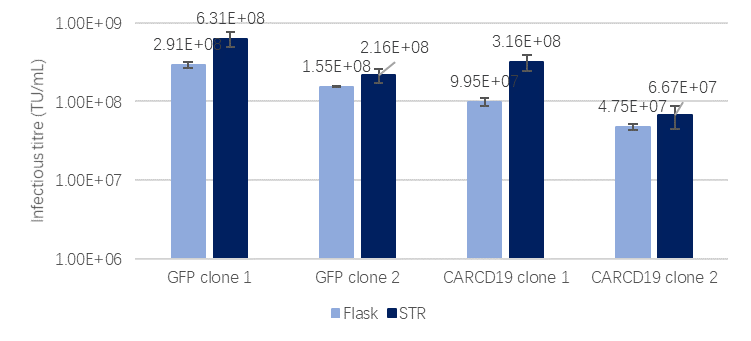
Fig 6. Scale-up study of LentiVV Producer Cell Line(GOI=GFP/Anti-CARCD19,Flask = E125 flask, 25 mL; STR = 1 L bioreactor)
Summary
WuXi Advanced Therapies's LentiVV stable cell line production platform offers an advanced, cost-effective, scalable, and robust solution for large-scale lentiviral vector manufacture. Both the LentiVV Packaging Cell Line and LentiVV Producer Cell Line achieve comparable or higher lentiviral vector yields compared to the four-plasmid transient transfection system. These consistent yields ensure a seamless transition from transient transfection to the LentiVV stable cell line production system.
Together with our standard Packaging and Producer cell lines offering, we will be able to develop Customized Packaging and Producer cell lines to support specific in-vivo and ex-vivo applications.
About WuXi Advanced Therapies (WuXi ATU)
As the advanced therapies business unit of WuXi AppTec, WuXi Advanced Therapies is a Contract Testing, Development and Manufacturing Organization (CTDMO) that offers integrated platforms to transform the discovery, development, testing, manufacturing, and commercialization of cell and gene therapies. Our services and solutions accelerate time to market and support customer programs around the world. For more information, please visit https://www.advancedtherapies.com.
|
